Project Summary
CoCure is a four-component resin system composed of Unsaturated Polyester Resin, Polyurethane, Isocyanate, and a Methyl ethyl ketone peroxide initiator. This resin system was developed by Dr. Ronnal Reichard and Scott Lewit at Structural Composites, a research and development company founded by the two in Melbourne, FL. This resin system has the capabilities to vastly change its properties by making minor changes to the ratio between each of the three polymers it contains. At one end of the spectrum, CoCure acts as a strong adhesive capable of bonding aluminum to composite materials. This allows for metals such as aluminum to be bonded easily to fiber reinforced polymers (FRPs), creating metal-hybrid composites. At the other end of CoCure's usage spectrum, it has the ability to act as a coating for both gel coats and molds. CoCure offers a higher elasticity than traditional gelcoats, leading to better crack resistance and weathering. The versatility of CoCure in its applications has gained the interest of companies that dominate the transportation industry. Wabash National, the largest semi trailer producer in North America, has adopted CoCure technology in their refrigerated trailers. Another titan in the transportation industry, Trinity Rail, the largest fleet of railcars in North America also uses CoCure technology as they transition into lighter alternatives to entirely steel railcars and move towards composite materials. Further uses for this technology is utilizing the enhanced crack resistance of CoCure on the hull of boats, increasing the lifespan of the gelcoat on boats ranging from small center-console fishing boats all the way to large and expensive yachts.
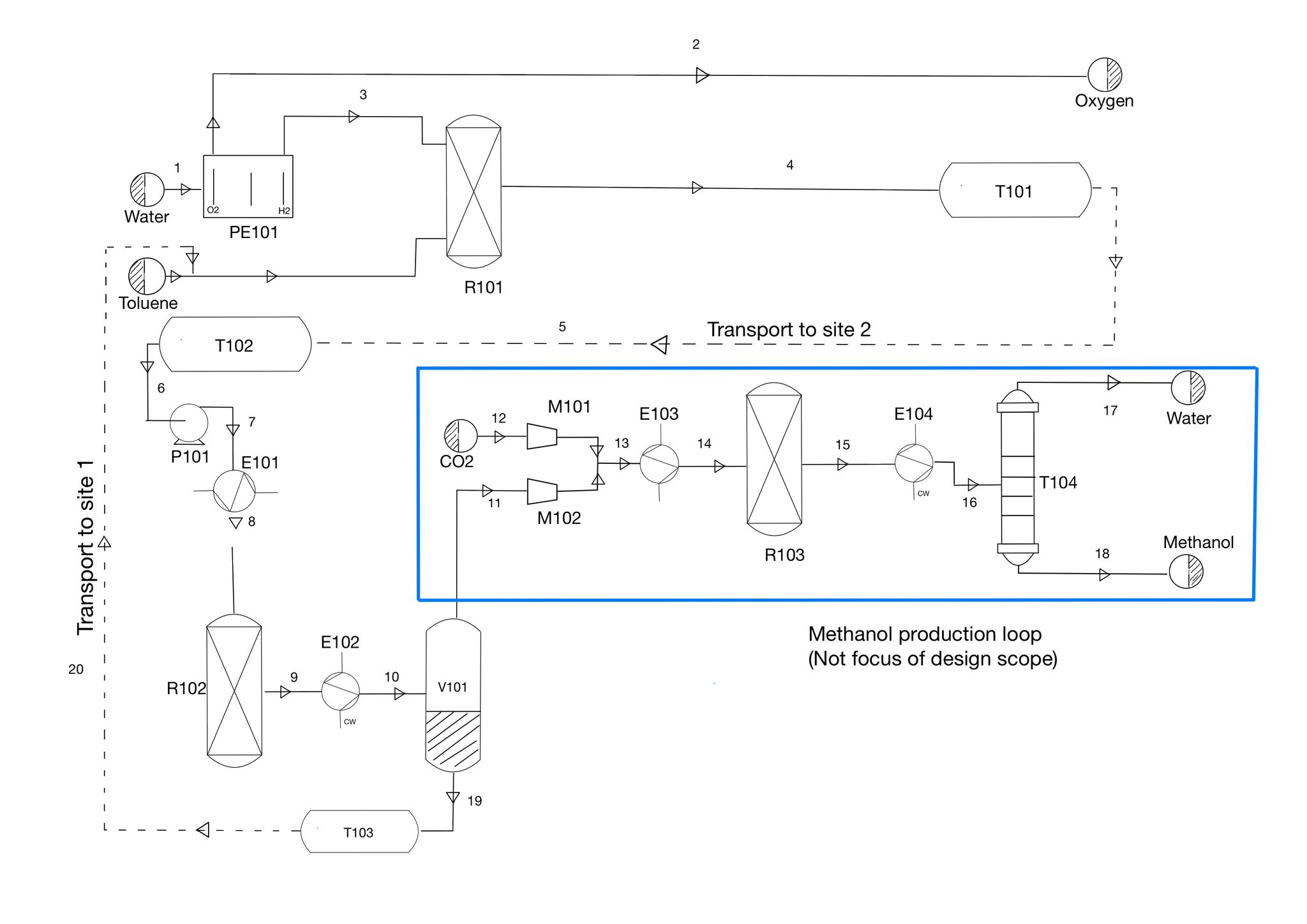
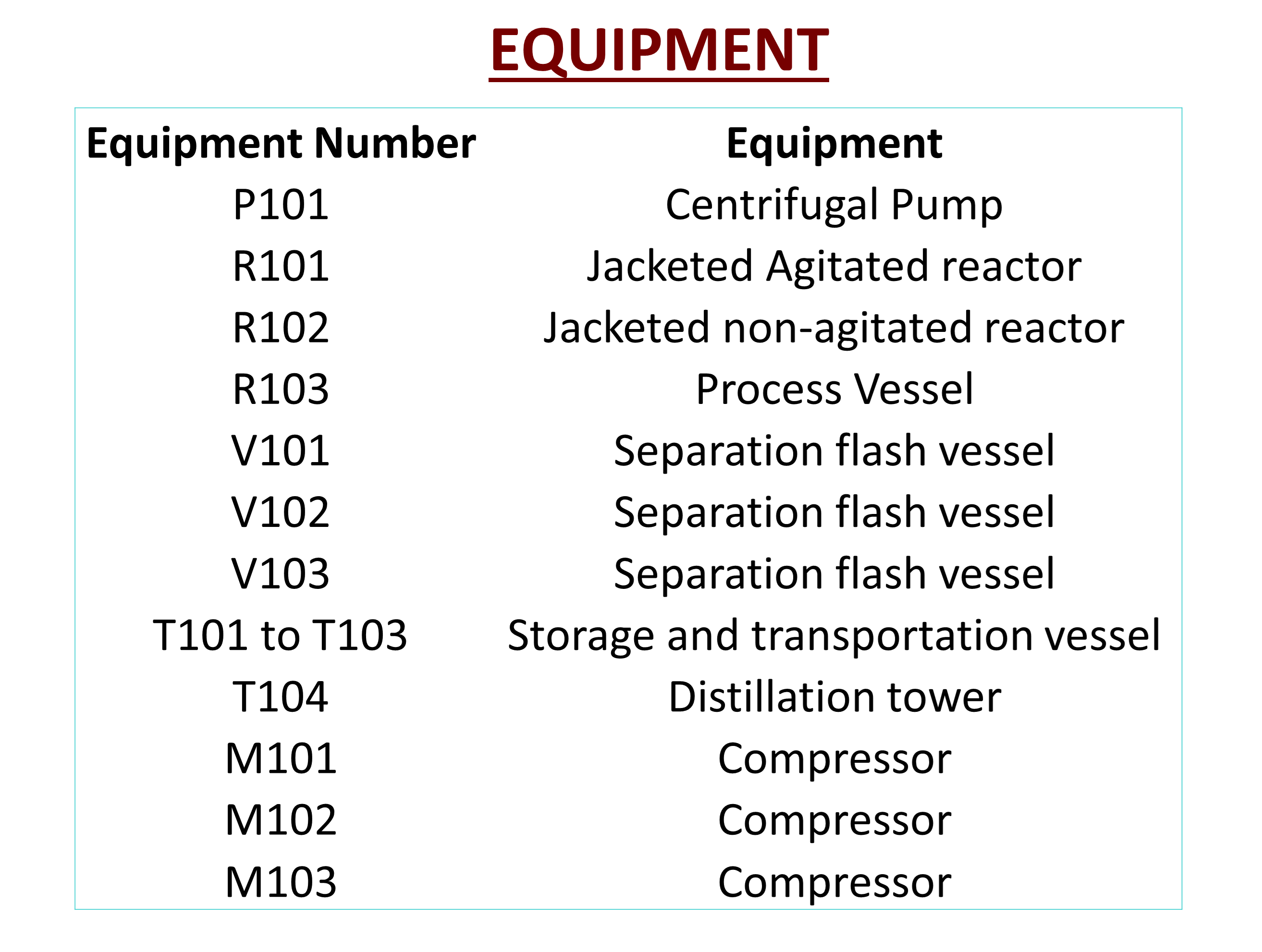
Although CoCure offers all of these benefits over traditional resin systems across many aspects of the composites industry, it is lacking in one major aspect, industrial application methods. CoCure in its liquid, uncured state is extremely viscous and reactive with air and water. This makes it difficult for CoCure to be used in existing resin spray systems used on an industrial scale. Structural Composites has tasked us to design and create a spray system that is capable of working across each matrix of CoCure with varying viscosities and chemical mixtures for near continuous industrial applications. To begin this process, it was necessary to first synthesize Unsaturated Polyester Resin, formally known as Poly(Propylene Glycol Maleate Phthalate)-Styrene Copolymer. This polymer makes up the majority of every combination of CoCure by mass and volume. This was done by reacting Propylene Glycol, Maleic Anhydride, and Phthalic Anhydride in an esterification reactor at 190 degrees Celsius. Separations of water, and unreacted base constituents happen in a string of flash vessels and a five-stage distillation column. This leaves 99.5% pure Unsaturated Polyester Resin to be used in the final production and spraying of CoCure.
From the synthesis and separation processes, Unsaturated Polyester Resin is mixed with Polyurethane and put under a blanket of Nitrogen gas. This is done because Isocyanate is extremely reactive with air and is one of the major issues that were experienced in previous attempts of creating a spray system for CoCure. Once Polyurethane and Polyester Resin are mixed and free of air, they are mixed with Isocyanate and heated to a temperature of 100 degrees Fahrenheit. This is done to address another major issue when developing previous iterations of this spray system, the viscosities of each component varies with temperature. By controlling the temperature of the process, it will allow for the pumping of each component to be more precise to the spray nozzle. Finally, at the spray nozzle of the system, the initiator that begins the curing process, Methyl ethyl ketone peroxide (MEKP925H), is added. From there, CoCure is ejected from the system to begin its exothermic reaction and begin curing.
Project Objective
The objective of this project is to design new pumping and mixing methodology for a four-component resin spray system that can handle the varying viscosities and flowrates required for each CoCure configuration.
Manufacturing Design Methods
Building from previous iterations of CoCure spray systems, looking at pumping, mixing, and the issues that arose with each gun, this project seeks to address CoCure composition issues that were found in the Wabash manufacturing facility in Little Falls, Minnesota. This project used Aspen Plus to model the synthesis of Unsaturated Polyester Resin and the mixing of each of the components of the final CoCure system.
Specification
This spray system should be capable of running continuously 24 hours a day, for 350 days in a year, maintaining a strict ratio between the levels of Polyester Resin, Polyurethane, and Isocyanate, accurate to within 1 gram of variance.
Analysis
Analysis was done on the accuracy of a magnetically coupled servo motor pump using hydraulic oil as a substitute for actual resin. Aspen Plus was used to simulate the mixing methodology for the spray system and the synthesis of Unsaturated Polyester resin.
Future Works
Future groups will continue to develop the physical spray system, building from the findings gained during the Aspen Plus simulation and from the tests run on the magnetically coupled servo motor pump.
Manufacturing Design Methods
Building from previous iterations of CoCure spray systems, looking at pumping, mixing, and the issues that arose with each gun, this project seeks to address CoCure composition issues that were found in the Wabash manufacturing facility in Little Falls, Minnesota. This project used Aspen Plus to model the synthesis of Unsaturated Polyester Resin and the mixing of each of the components of the final CoCure system.
![]()