Tissue engineering is a multidisciplinary field (physics, chemistry, biology, engineering) that employs a combination of cells, scaffolds, and bioactive molecules to generate functional tissue grafts for the repair, replacement, or regeneration of diseased tissues. Biomaterials are biocompatible natural or synthetic materials that interact with biological systems and used for prosthetic, diagnostic, therapeutic, or storage applications. In the tissue engineering realm, biomaterials are used to engineer a temporary framework (scaffold) that resembles the native tissue and provides a support structure to facilitate cell adhesion, proliferation, differentiation, and tissue regeneration.
Dr. Vipuil Kishore's Functional Biomaterials and Tissue Engineering laboratory in the chemical engineering program at Florida Tech has several ongoing research projects on the development of collagen-based biomimetic scaffolds for tissue engineering applications. Biofabrication techniques such as 3D printing, freeze-casting and electrochemical alignment are used to process collagen solutions and generate scaffolds that mimic the physicochemical properties (composition, structure, mechanics) of native tissue extracellular matrix. These biomimetic scaffolds can provide the essential physical and biochemical cues to direct cell response and guide functional tissue regeneration.
Research in Dr. Manolis Tomadakis's group is focusing on developing computational models to understand transport through core-sheath fiber systems for biomedical and biological systems.
Some of the projects that are currently ongoing in the chemical engineering program in the areas of biomaterials and tissue engineering are briefly described below.
Kishore Lab | Dr. Vipuil Kishore
Anterior Cruciate Ligament (ACL) injuries are highly common in sport athletes and elderly people affecting more than 150,000 Americans each year and costing an estimated 500 billion dollars in healthcare costs. Current treatment options with artificial grafts are associated with suboptimal healing, repetitive graft failure at the bone-ligament interface, and poor clinical outcome.
In this NIH funded research project, Kishore lab is employing advanced technologies such as Raman spectroscopy and 3D bioprinting to generate biomimetic bioglass gradient integrated collagen matrices (BioGIMs) that is compositionally similar to the bone-ligament transition region with a goal to improve graft integration by promoting cell migration, tissue-specific cell differentiation, and new matrix production. -956x206.jpg)
Kishore Lab | Dr. Vipuil Kishore
Extrusion-based 3D printing is a layer by layer technique that allows for the fabrication of custom-designed complex tissue architectures. However, suboptimal resolution of the extrusion printing technique offers little control over the microscopic features of the 3D construct. These microscopic features (e.g., pore size, pore morphology) are known to have a profound effect on cell migration, cell-cell interaction, proliferation, and differentiation.
In this project currently ongoing in Kishore lab, FRESH-based 3D printing and freeze-casting is combined in a Freeze-Fresh (FF) approach to generate microporous collagen scaffolds. These microporous scaffolds can promote greater cell infiltration, cell-cell interaction, and vascularization, and hence can be used in a variety of different tissue engineering applications.
-761x204.jpg)
Kishore Lab | Dr. Vipuil Kishore
Coronary artery disease (CAD) due to atherosclerosis (build up of fat and cholesterol on blood vessel wall) is the leading cause for deaths in the United States accounting for 1 in 7 deaths each year. Bypass surgery using autologous grafts is the current gold standard but these grafts are not available in > 30% of patients. Use of synthetic grafts is limited to the replacement of large-diameter vessels.
Kishore lab is working on a project to develop a biomimetic tissue-engineered vascular graft (TEVG) for replacement of diseased small-diameter arteries (< 6 mm). An electrochemical fabrication method is employed to densify pure collagen solution and form a smooth tubular lumen. Insoluble elastin incorporated electrochemically aligned collagen fibers are circumferentially wound around the lumen to generate a biomimetic bi-layered scaffold that resembles the compositional and structural aspects of the native vessel. Current work is focusing on designing perfusion-based bioreactor systems to co-culture smooth muscle cells and endothelial cells on the bi-layered scaffold and assess scaffold performance -975x231.jpg)
Kishore Lab | Dr. Vipuil Kishore
Corneal disease is the second major cause for blindness. Lack of supply of donor corneas has prompted researchers to develop alternative strategies for corneal repair. The electrochemical fabrication approach is employed to densify collagen solution and generate highly transparent dense collagen matrices for use in corneal applications. The long term goal of this project in Kishore lab is to generate a multi-layered cell-laden collagen scaffold towards the development of a fully tissue engineered cornea as a viable alternative to donor corneas.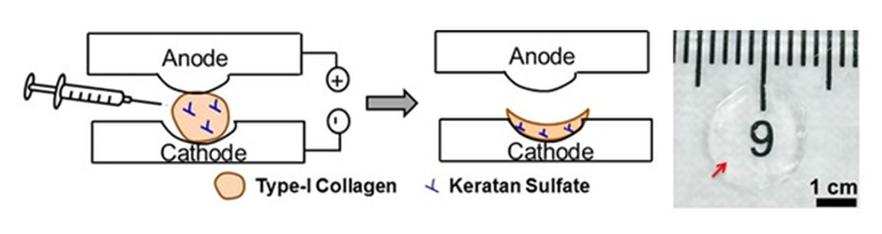
Core-sheath fiber systems are three-component media consisting of ordered or randomly oriented fibers, each sheathed by an annular layer of a different material, with the fiber bundle surrounded by a fluid or solid matrix phase. Such systems find numerous applications across a broad spectrum of scientific R&D fields, primarily in biomedical and biological systems.
A number of investigations of such media have focused on their use as tissue engineering scaffolds for tissue repair and regeneration, driven in part by the scaffold potential for sustainable, controllable, and efficient release of bioactive agents such as drugs, proteins, and enzymes. Studies have demonstrated the potential of such scaffolds in applications involving local cancer treatment, stem cell attachment on biocompatible nanofibers, endovascular stents preventing restenosis, manipulation and segregation of viruses and bacteria, electric field effects on myelinated nerve fibers, and nerve regeneration. Core-shell fiber systems also capture the macro-geometry characteristics of cardiac papillary muscle and chordae tendinae (heart strings), essential for the proper function of the mitral valve. Non-biological applications of core-sheath fibrous media include energy storage composites, thermionic fuel elements, thermally bonded fabric filters, optical fiber cables, hypersonic engine seals, radar absorbing and infrared camouflage, and nanofibers in electronics, chemical sensors, and other nanotechnology systems.
In this study, numerical methods were developed for estimating effective mass, energy, and momentum transport properties of core-shell fibrous biomaterials of various core, shell, and matrix volume fractions and phase conductivities. Such effective transport properties of core-sheath fiber structures are key parameters affecting the performance of such media in most of the above applications. For instance, modeling of the electrical activity in cardiac tissue requires knowledge of its electrical conductivity. Both the electrical and thermal conductivity of the heart tissue are needed to improve understanding of cardiac radiofrequency ablation, an interventional technique employed in the treatment of cardiac arrhythmias. The effective diffusivity and thermal conductivity of core-shell fibrous tissue-engineering scaffolds control the rate of thermally regulated delivery of drugs and other bioactive agents, and their electrical conductivity becomes of essence too when electroactive polymers are used as scaffold materials.


 Give to Florida Tech
Give to Florida Tech 